Our Research
We are passionate about combining our unique access to the human brain with emerging genomic and translational technologies to re-envision and advance cerebrovascular medicine.
Topics
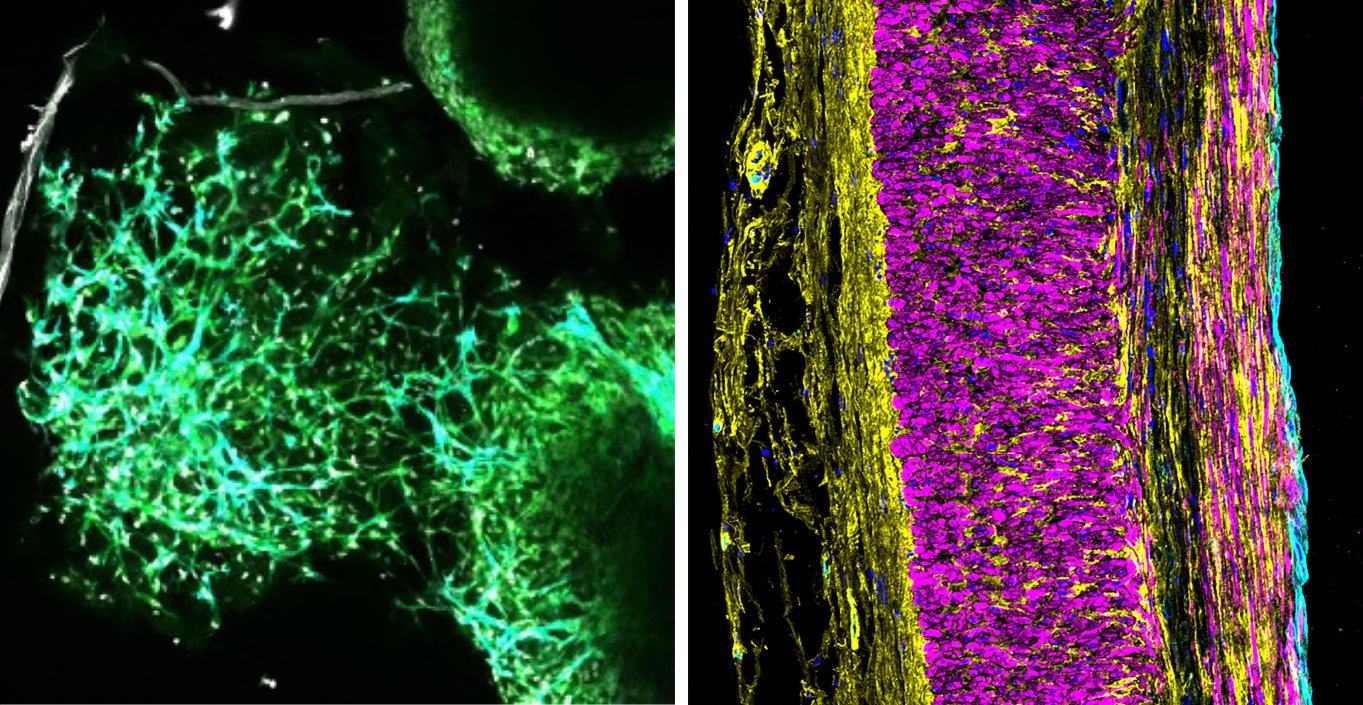
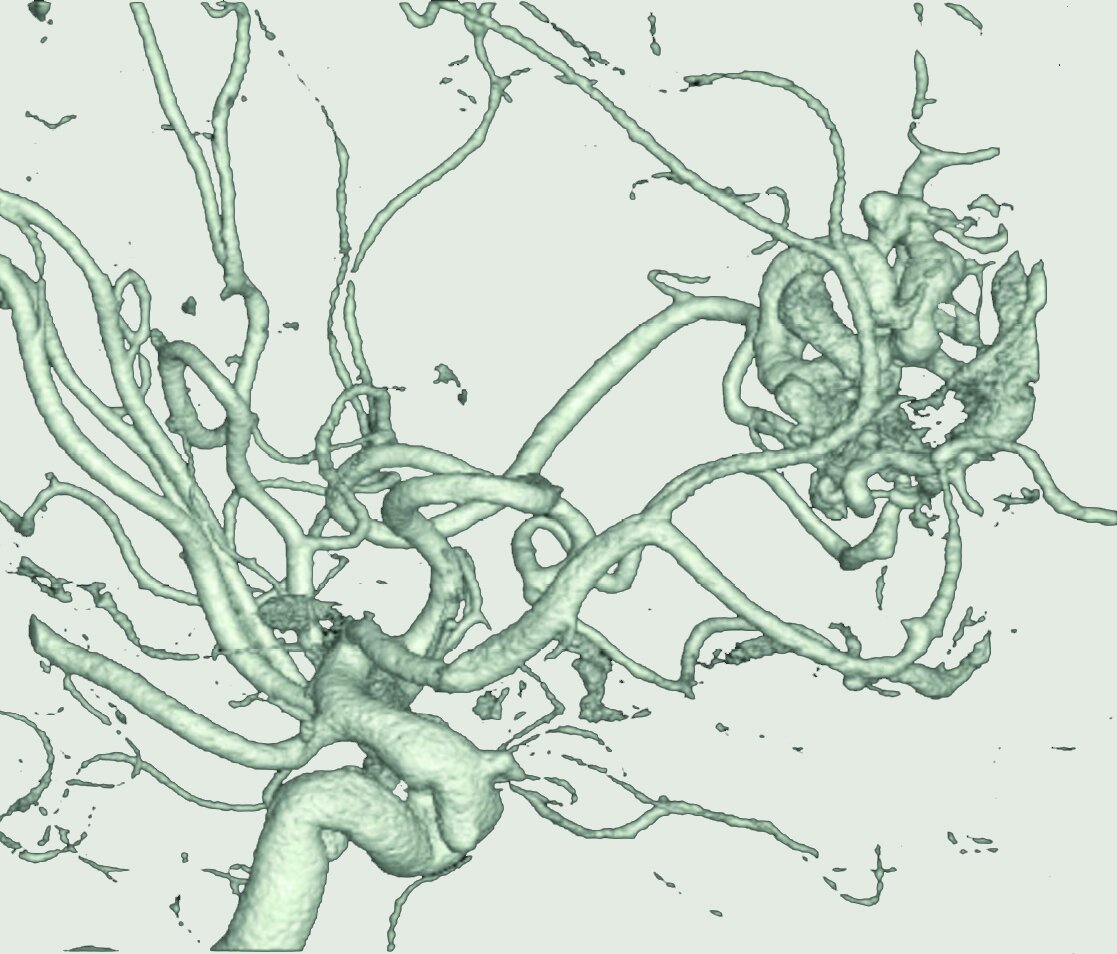
Cell-specific genomic contributors to human vascular malformations
Using single-cell genomics and spatial transcriptomics, we recently assembled a cell-resolution molecular map of the human brain’s vasculature. By applying this approach to human arteriovenous malformations (AVMs), we discovered molecular hallmarks of abnormal vascular patterning and cellular contributors to hemorrhagic stroke. We are now investigating the upstream regulatory mechanisms which cause vascular malformations to form or progress and developing cell-specific precision therapeutics to prevent AVMs from bleeding.
Functional genomics & regenerative vascular cell engineering to restructure the cerebrovasculature
Depletion of cerebrovascular support cells, known as mural cells, results in fragility and hemorrhagic stroke. However, there are no means to replenish these cell populations. Using induced pluripotent stem cells (iPSCs) and vascular organoids, we are now engineering the cerebrovasculature in vitro to investigate mechanisms of mural cell depletion with functional genomics and to devise means of cell-based replenishment to stabilize the diseased vasculature.
Non-invasive human cerebrovascular molecular profiling
Vascular malformations and aneurysms are not amenable to traditional biopsies. To translate our genomics approaches, we recently developed a new technology to extract and molecularly profile brain endothelial cells in patients without the need for surgery, called endoluminal biopsy. Using this technology, we are now studying molecular attributes of the human cerebrovasculature in vivo. We are also investigating the molecular heterogeneity of arteriovenous malformations and aneurysms to re-classify them biologically and explain differences in behavior or response to precision-based therapeutics.
Enhanced delivery of brain therapeutics
The blood-brain barrier (BBB) is a unique property of the cerebrovasculature and tightly regulates molecular and cellular exchanges between the brain and circulation. While the BBB is needed for brain homeostasis, it serves as a perpetual obstacle for delivering brain therapeutics. Focused ultrasound is an emerging non-invasive technology to selectively disrupt the BBB to enhance delivery of therapeutics to the brain. Using novel primary tissue and organoid models, we are now investigating how to harness this technology to deliver next generation vascular- and brain-targeted therapeutics.